PRODUCT IDENTIFICATION
1.2 - 2.8
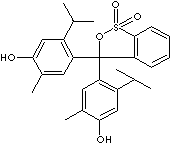
EINECS NO.
2914.70.4000
Thymolsulfonephalein;
Thymolsulfonephthalein; Thymolsulfophthalein; Thymolsulphonphthalein; Thymosulfonphthalein; Thymolblau; Azul de timol; Bleu de thymol; 4,4'-(3H-2,1-Benzoxathiol-3-ylidene)bis(5- methyl-2-(1-methylethyl)phenol S,S-dioxide; 4,4'-(1,1-Dioxido-3H-2,1-benzoxathiol -3-ylidene)bis(5-methyl-2-(1-methylethyl)phenol; 6,6'-(3H-2,1-Benzoxathiol-3-ylidene)dithymol S,S-dioxide; Other RN: 2877-93-2; 18611-66-0; 264233-99-0; 313494-17-6; 354556-94-8; 384814-32-8; 400864-47-3; 405271-03-6; 551921-90-5; 672309-78-3; 720671-81-8
SMILES
CLASSIFICATION
DYES / INDICATOR /
PHYSICAL AND CHEMICAL PROPERTIES
2.05E-10 (cm3/molecule-sec at 25 C Atmospheric )
REFRACTIVE INDEX
APPLICATIONS
- alpha-Naphthol phthalein (CAS #: 596-01-0): from pH 7.3 (pinkish-yellow) to pH 8.7 (greenish-blue)
- Bromocresol green (CAS #: 76-60-8): from pH 3.8 (yellow) to pH 5.4 (blue)
- Bromcresol Purple (CAS #: 115-40-2): from pH 5.2 (yellow) to pH 6.8 (purple)
- Bromochlorophenol Blue (CAS #: 2553-71-1): from pH 3.2 (yellow) to pH 4.8 (blue)
- Bromocresol purple, sodium salt (CAS #: 62625-30-3): from pH 5.2 (yellow) to pH 6.8 (purple)
- Bromophenol blue (CAS #: 115-39-9): from pH 3.0 (yellow) to pH 4.6 (blue)
- Bromophenol blue, sodium salt (CAS #: 62625-28-9): from pH 3.0 (yellow) to pH 4.6 (blue)
- Bromopyrogallol Red (CAS #: 16574-43-9): suitable for complexometric or chelometric titrations
- Bromothymol blue (CAS #: 76-59-5): from pH 6.0 (yellow) to pH 7.6 (blue)
- Bromothymol blue, sodium salt (CAS #: 34722-90-2): from pH 6.0 (yellow) to pH 7.6 (blue)
- Bromoxylenol Blue (CAS #: 40070-59-5): from pH 6.0 (yellow) to pH 7.6 (blue)
- Chlorophenol Red, water soluble (CAS #: 123333-64-2): from pH 4.6 (yellow) to pH 7.0 (purple)
- Cresol Red (CAS #: 1733-12-6): from pH 7.0 (orange) to pH 8.8 (purple)
- Fluorescein (CAS #: 2321-07-5)
- Fluorescein sodium (CAS #: 518-47-8):
- Glycinethymol Blue (CAS #: 3810-63-7):
- Iodophthalein sodium (CAS #: 2217-44-9)
- m-Cresol purple (CAS #: 2303-01-7): from pH 7.4 (yellow) to pH 9.0 (purple)
- Methylthymol blue, sodium salt (CAS #: 1945-77-3): suitable for complexometric or chelometric titrations
- o-Cresolphthalein (CAS #: 596-27-0): from pH 8.2 (colorless) to pH 9.8 (red)
- o-Cresolphthalein Complexone (CAS #: 2411-89-4): suitable for complexometric or chelometric titrations
- Phenolphthalein Complexone (CAS #: 25296-54-2):
- Phenol Red (CAS #: 143-74-8): from pH 6.8 (yellow) to pH 8.2 (red)
- Phenol Red Sodium Salt (CAS #: 34487-61-1): from pH 6.8 (yellow) to pH 8.2 (red)
- Phenolphthalein (CAS #: 77-09-8): from pH 8.5 (colorless) to 9.0 (red)
- p-Xylenol Blue (CAS #: 125-31-5):
- Acid range: from pH 1.2 (red) to pH 2.8 (yellow)
- Alkali range: from pH 8.0 (yellow) to pH 9.6 (purplish-blue)
- Pyrocatechol violet (CAS #: 115-41-3): suitable for determination of many metals (complexometric or chelometric titrations) particularly rapid for tin
- Pyrogallol Red (CAS #: 32638-88-3):
- Thymol Blue, sodium salt (CAS #: 62625-21-2):
- Acid range: from pH 1.2 (red) to pH 2.8 (yellow)
- Alkaline range: from pH 8.0 (yellow) to pH 9.2 (blue)
- Thymolphthalein Complexone (CAS #: 1913-93-5): suitable for complexometric or chelometric titrations
- Thymolphthalein (CAS #: 125-20-2): from pH 8.8 (colourless) to pH 10.5 (blue)
Phthalein is the moiety in a dye which shows color changes depend on particular hydrogen ion concentrations. Certain phthaleins (e.g. phenolphthalein) have a cathartic action.
APPEARANCE
95.0% min
LOSS ON DRYING
3.0% max
CLARITY OF SOLUTION
pass
(alkaline range): from pH 8.0 (yellow) to pH 9.6 (blue)
BIOLOGICAL STAINS, INDICATORS